On February 17, 2023, the U.S. Food and Drug Administration (FDA) approved intravitreal pegcetacoplan (brand name Syfovre), the first-ever treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD). Texas Retina Associates was involved in the Phase III clinical trial (OAKS) that led to the approval and is also part of the extension study (GALE) that will evaluate the long-term impact of this treatment.
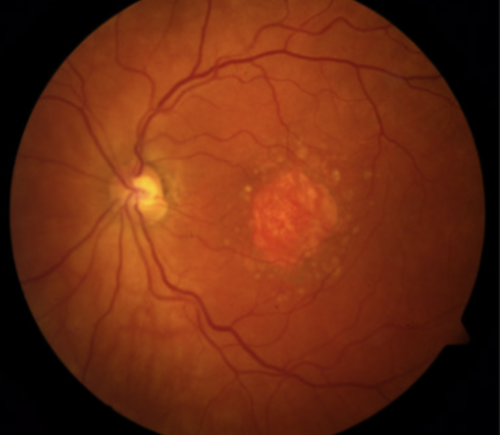
Understanding Geographic Atrophy (GA)
Approximately 20% of patients with AMD will develop geographic atrophy (GA), a leading cause of blindness impacting more than one million people in the United States. A progressive disease, it is considered an advanced form of AMD and is caused by the growth of lesions that destroy the retinal cells responsible for vision (atrophy). Upon examination by an ophthalmologist or retina specialist, these areas of atrophy often look like a map which led to the term “geographic atrophy.”
How Intravitreal Pegcetacoplan (Syfovre) Works
Administered as an intravitreal injection, pegcetacoplan (Syfovre) was designed to regulate activation of the complement cascade, part of the body’s immune system that leads to the onset and progression of geographic atrophy (GA). The two clinical trials that led to approval of this new treatment, OAKS and DERBY, demonstrated up to a 36% reduction in geographic atrophy (GA) lesion growth over a 24-month period.
“This is a huge milestone in the field of retinal ophthalmology that has been more than a decade in the making,” explains Ashkan Abbey, MD, Texas Retina Associates’ Director of Clinical Research for Dallas. “Geographic atrophy can significantly impact the quality of life and independence of the patients who suffer from it. Now we have a tool to slow its progression and help protect their vision.”
Syfovre is expected to be commercially available in March.
Ongoing Research on Additional Potential Treatments for Geographic Atrophy (GA)
In addition to the OAKS study that led to the FDA approval of intravitreal pegcetacoplan (Syfovre), Texas Retina Associates has been involved in a number of other Phase II and III clinical trials to test potential new treatment options for geographic atrophy (GA). This includes the Phase III GATHER1 and GATHER2 trials of another drug called avacincaptad pegol (Zimura). The manufacturer of that drug is expected to submit a New Drug Application to the FDA by the end of the first quarter of 2023.